Mitotic Entry
Understanding the molecular mechanisms that control the G2/M transition and entry into mitosis in mammalian cells.
Live imaging of cells entering mitosis
Research Overview: The G2/M Transition; How Cells Decide to Divide
Every time a cell divides, it faces a critical decision point: is everything ready for the complex choreography of mitosis? This decision happens at the G2/M transition — a cellular checkpoint that ensures mitosis is triggered at exactly the right time.
The Molecular Switch
At the heart of this transition lies CDK1, a master regulator that acts like a molecular switch. When activated, CDK1 rapidly modifies over 1,000 proteins throughout the cell, triggering a cascade of events:
- Chromosomes condense into their familiar X-shaped structures
- The nuclear envelope dissolves, allowing access to the genetic material
- The mitotic spindle assembles to pull chromosomes apart
Our lab investigates how cells flip this switch with such remarkable precision. We study the intricate feedback loops that amplify CDK1 activation, ensuring the transition is both rapid and irreversible once initiated.
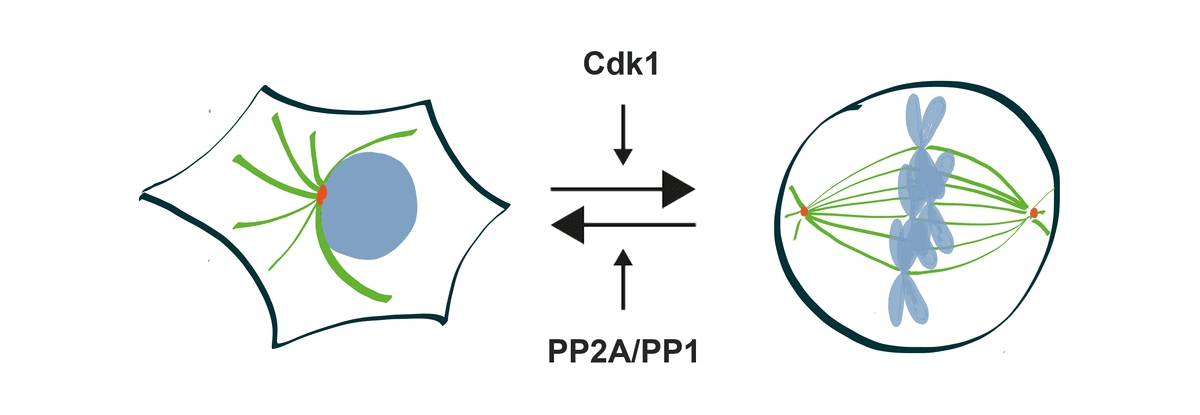
Two Pathways, One Decision: How Cells Commit to Division
Our research has revealed that mitotic entry is not controlled by a single pathway, but by two interconnected regulatory systems that work together to ensure cells divide at exactly the right time. Understanding this dual control mechanism provides fundamental insights into one of biology's most critical decisions.
The Bistable Switch System
We discovered that mitotic entry operates like two interconnected molecular switches: one that activates CDK1 and another that simultaneously inactivates the opposing phosphatase PP2A:B55. This dual-switch architecture creates “bistability” — cells exist in one of two stable states (interphase or mitosis) with distinct thresholds for switching between them.
Using specially engineered cells where we could precisely control CDK1 activity, we showed that eliminating either switch alone still allows mitotic entry, but removing both completely abolishes the cell's ability to maintain distinct cell cycle states.
→ Read our Current Biology paper on bistable mitotic switches
Cyclin Specialization: Division of Labor in Mitosis
While both cyclin A and cyclin B can activate CDK1, we found they have surprisingly distinct roles. Cyclin A acts as the “trigger” — it initiates the mitotic program and is absolutely required for cells to enter mitosis. Remove cyclin A, and cells remain permanently stuck in G2 phase.
Cyclin B, however, can be bypassed during mitotic entry through the parallel Greatwall-PP2A pathway. But once mitosis begins, cyclin B becomes essential for nuclear envelope breakdown and for phosphorylating the specific subset of proteins needed to complete cell division. This reveals an elegant division of labor: cyclin A initiates mitosis, cyclin B executes it.
→ Read our EMBO Journal paper on cyclin A and B specialization
Key Research Areas
- • CDK1 activation mechanisms
- • Greatwall kinase regulation
- • Phosphatase inhibition
- • Crosstalk between replication and mitotic entry
- • Discovery of novel mitotic modulators
Current Projects
- • Single-cell dynamics of mitotic entry
- • Proteomics of mitotic phosphorylation
- • Genetic screening for mitotic modulators
- • Mathematical modeling of G2/M transition
Methodological Approaches
Cell Biology
- • Live cell microscopy
- • High-content single-cell imaging
- • Degron tagging
- • CRISPR optimization
Biochemistry
- • Kinase assays
- • Mass spectrometry
- • Protein purification
- • Structure/Function studies
Computational Biology
- • High content imaging pipelines
- • 4i cyclic immunofluorescence
- • Cell tracking and classification
- • Gene dependency analysis